Medical related industry clusters
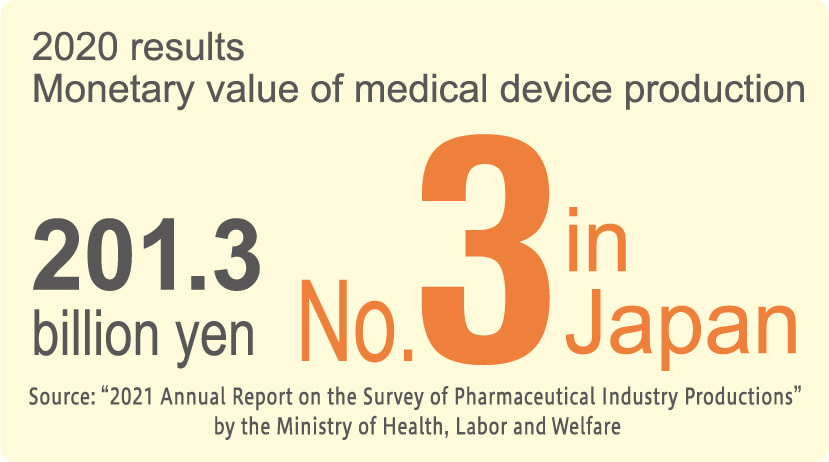
Fukushima Prefecture is one of the best "medical equipment producing prefectures" in Japan. Numerous production bases of large medical equipment manufacturers are located here. Furthermore, small and medium-sized businesses that provide strong support for this production have accumulated in the prefecture, forming the largest cluster of such companies nationwide, which makes Fukushima the No.1 prefecture in the Shipment value of medical equipment components, etc., in Japan.
Next-Generation Medical Industry Accumulation Project
Fukushima has been promoting the project to accumulate medical-related industries since FY2005 typified by the research and development of an industry-academia-government collaboration. With the Fukushima Translational Research Center and Fukushima Medical Device Development Support Center opened in 2016 serving as a hub, we are working towards further development and promotion of the medical-related industry field which brings out the best of Fukushima's strength in manufacturing.
Cluster situation of medical device industry
Medical care - Industry Translational Research Center
(Fukushima Global Medical Science Center, Fukushima City)
The center implements multi-faceted development support for new therapies, diagnostic drugs, test reagents, etc. for various diseases such as infectious diseases and cancers. It converts and offers clinical samples specialized for aiding development of drugs, etc. (specimens and clinical information) into analytical data, processed matter, and bio-evaluation systems, as well as promotes practical application of natural human antibodies using its independently developed protein microarray technology.

Fukushima Medical Device Development Support Center
(Koriyama City)
The center is the first public testing organization in Japan that can carry out biological testing using large animals and electrical, physical and chemical safety testing, etc., to offer one-stop support from development to commercialization. Being able to carry out such safety evaluations in Japan means shorter development time-frames as well as lower costs. Together with this, the center also carries out business matching and consulting, as well as training of health care workers utilizing developed and prototype products, and offers integrated assistance right from development through to commercialization of medical devices.

The center's four functions
Safety evaluation function
ISO/IEC17025 certified. Supporting standards/regulations of GLP/AAALAC. The center evaluates the safety of medical devices with non-clinical test.
Consultation/Information provision function
Supporting to launch a business in a medical device field to its commercialization comprehensively. Personal assistance arrangement for each company contributes smooth development and improvement of medical devices.
Development and human resource training function
In a similar environment to clinical site, providing training for various procedures. The place is available to promote a new product of medical device manufactures.
Matching function
Speeding up of commercialization is achievable because of "Fukushima". The center supports expansion of business opportunities for companies and promotes product development.
Potential data
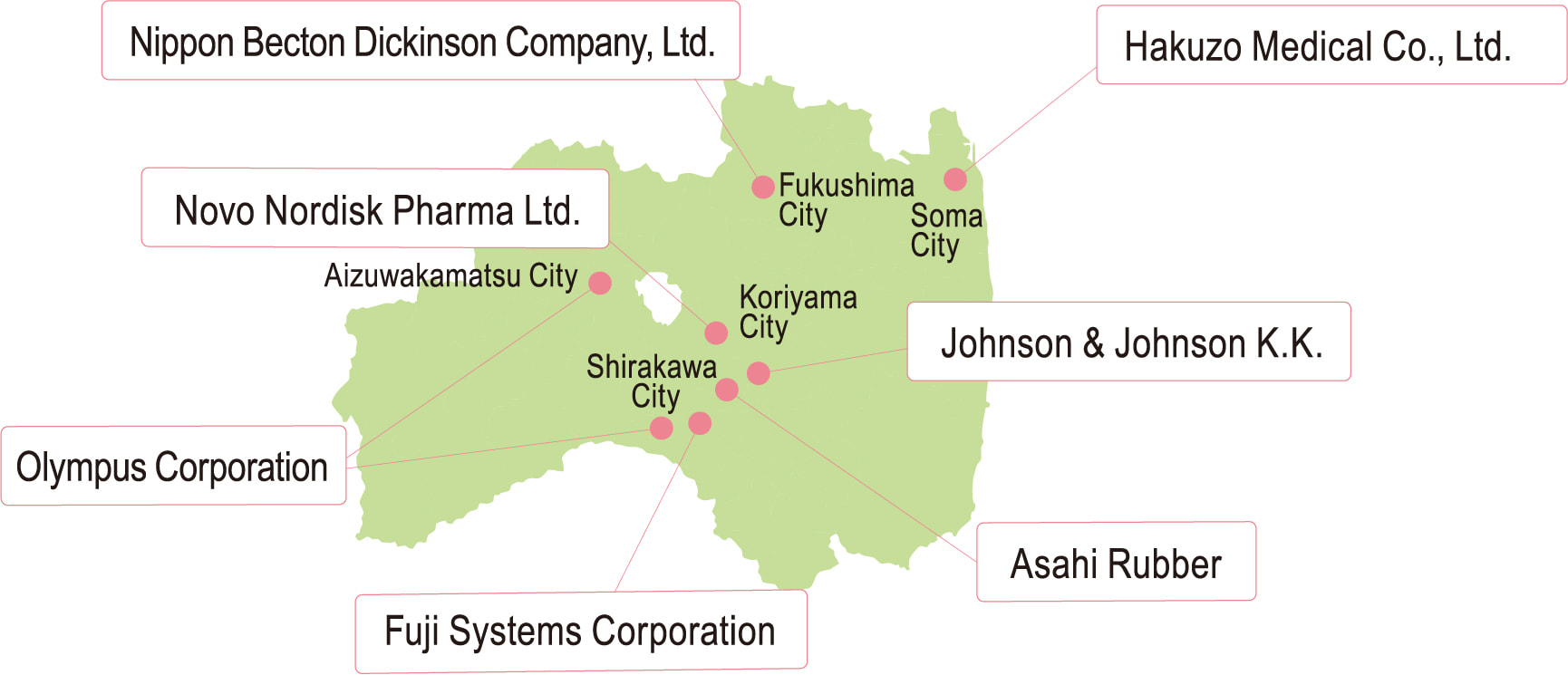
Fukushima is home to an Olympus production plant, and approximately 70% of the world's digestive endoscopes are produced within the prefecture. Fukushima also has important importing and production bases of major American medical equipment manufacturers like Johnson & Johnson and Nippon Becton Dickinson, and around 70 medical device manufacturers including Novo Nordisk Pharma, Hakuzo Medical, Fuji Systems operate here.
INDEXGet to know Fukushima
03Challenge